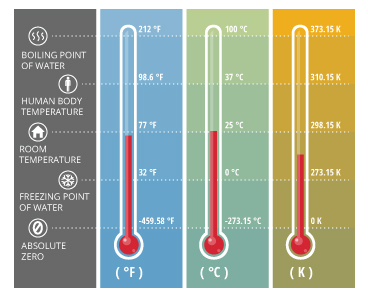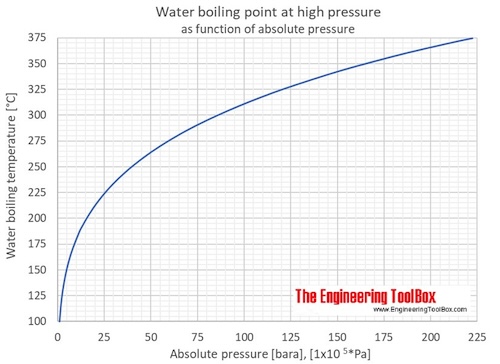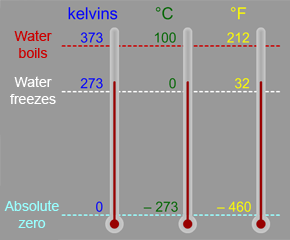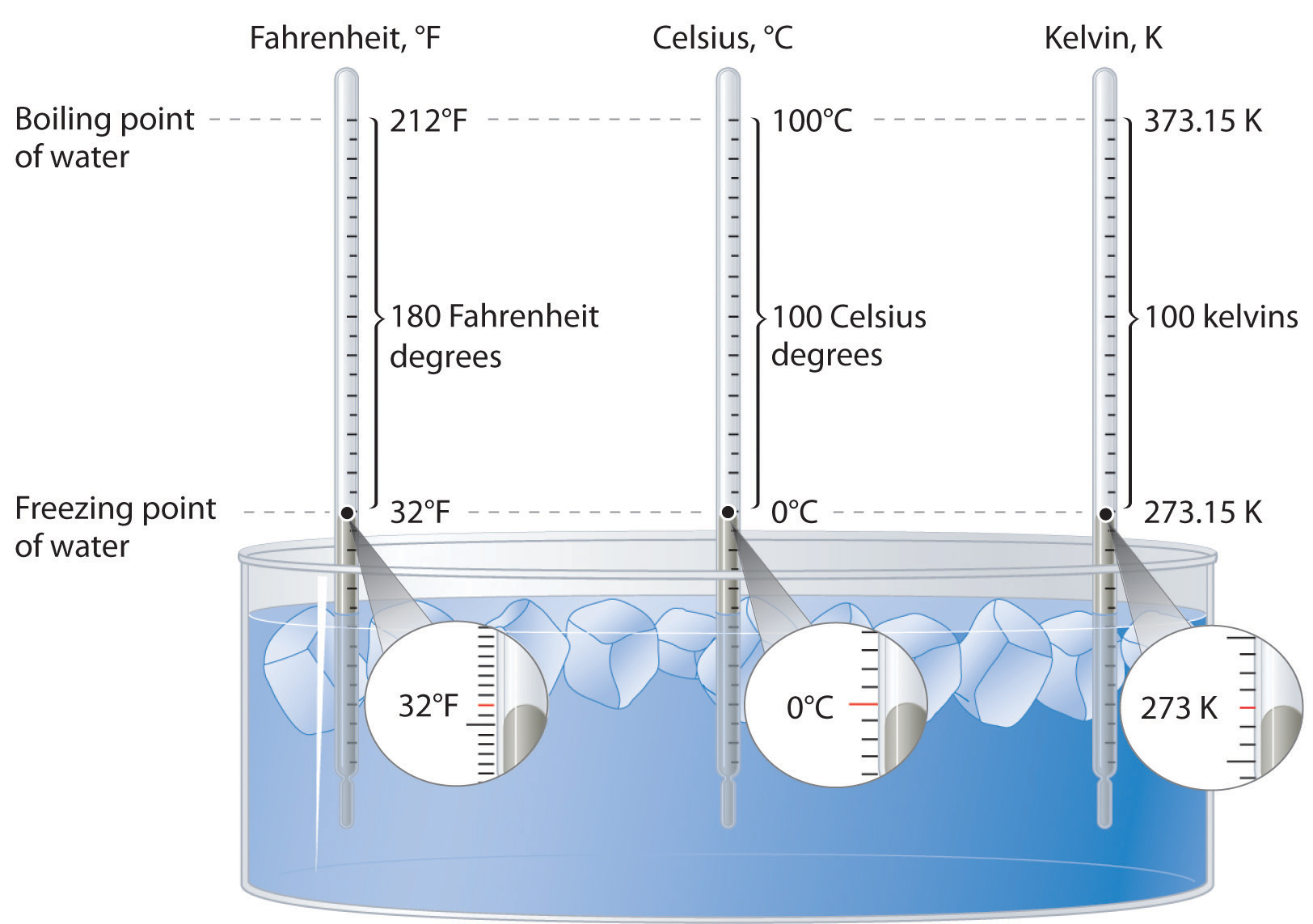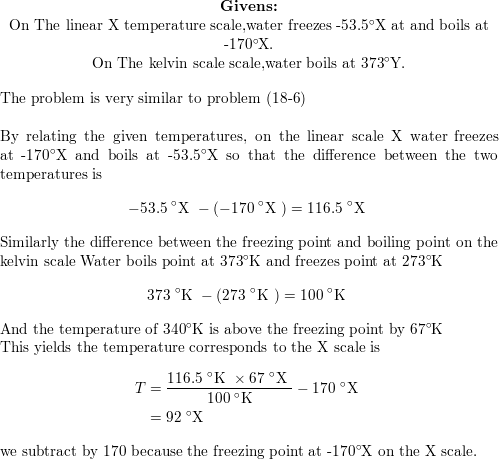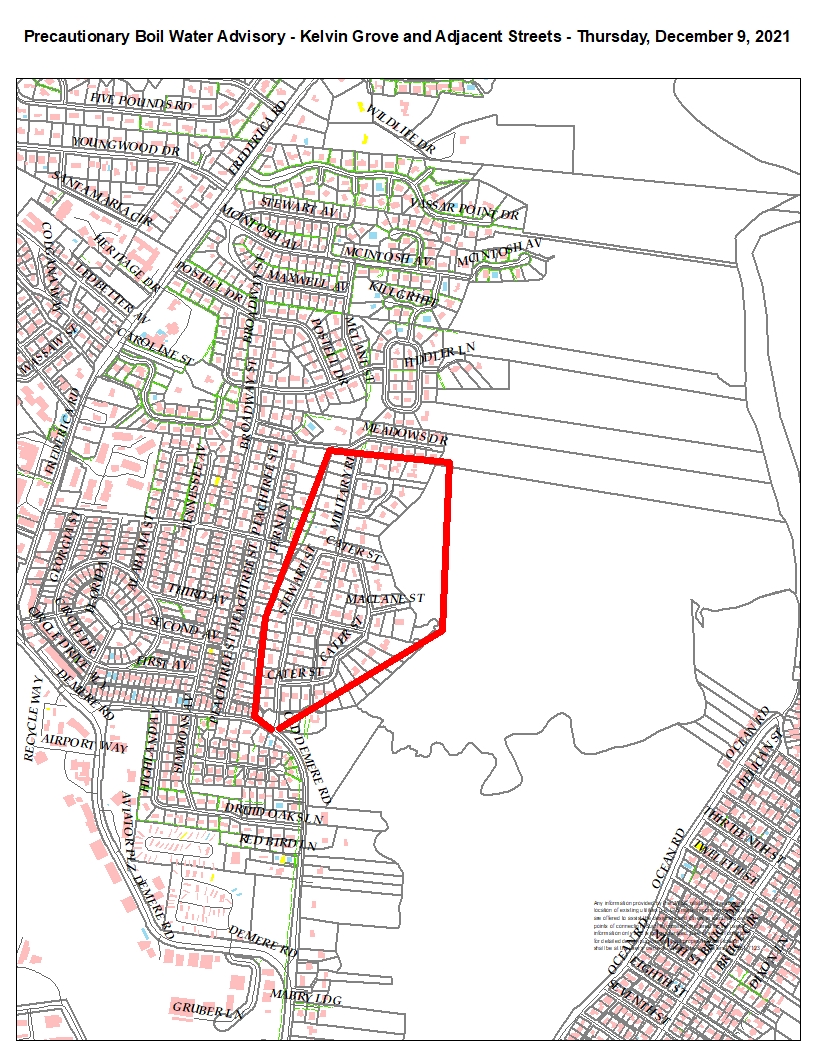
Precautionary Boil Water Advisory – Kelvin Grove Area on SSI – December 9, 2021 – Brunswick-Glynn County Joint Water & Sewer Commission

Normal boiling point of water is 373 K. Vapour pressure of water at 298 K is23 mm enthalpy of va... - YouTube
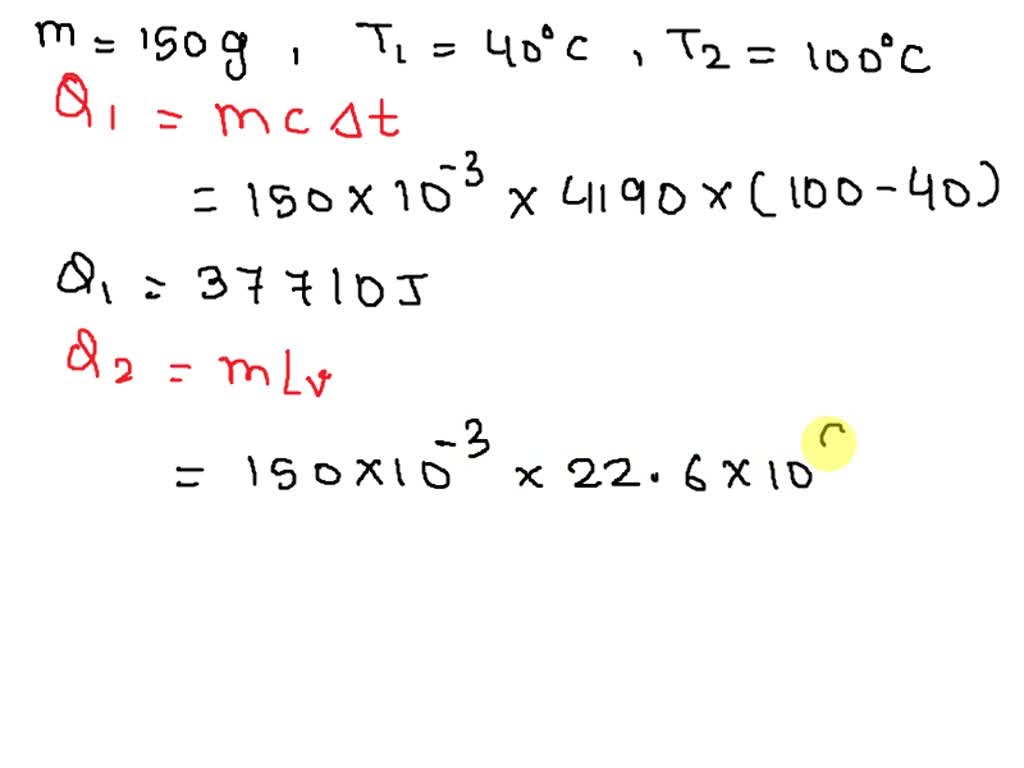
SOLVED: What minimum heat is needed to bring 150 g of water at 50 ∘C to the boiling point and completely boil it away? The specific heat of water is 4190 J/(kg⋅K)

18 g of glucose, C6H12O6, is dissolved in 1 kg of water in a saucepan.At what temperature will water boil at 1.013 bar? Kb for water is 0.52 K kg mol 1

Water is brought to boil under the pressure of 1.0 atm . When an electric current of 0.50 A from a 12 V supply is passed for 300 s through resistance in