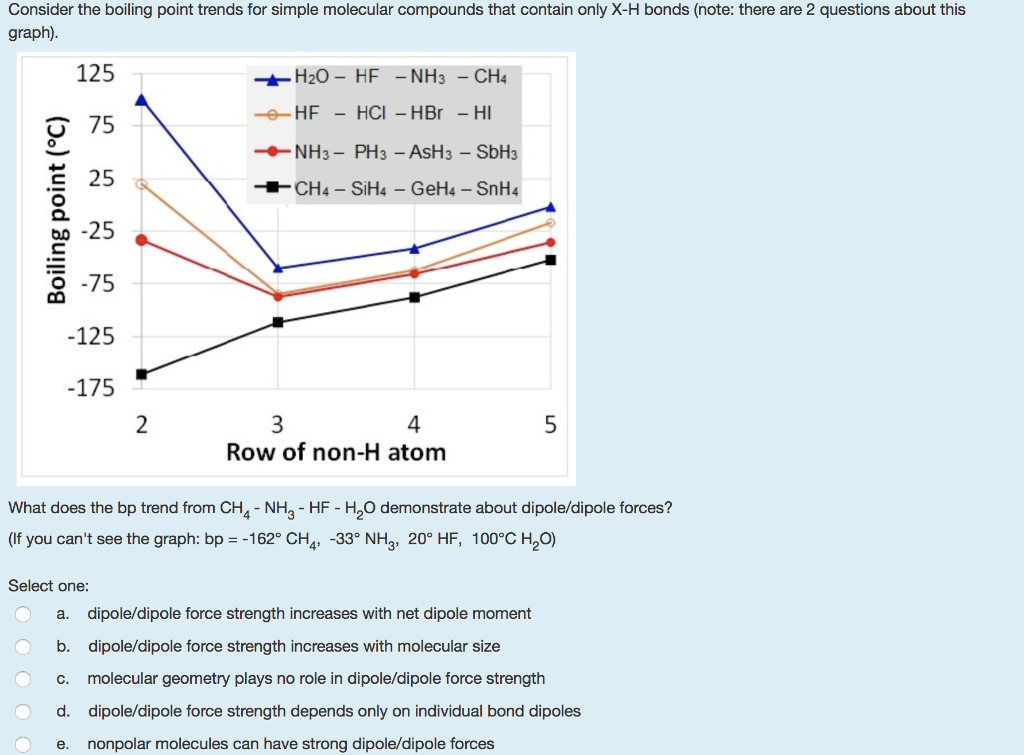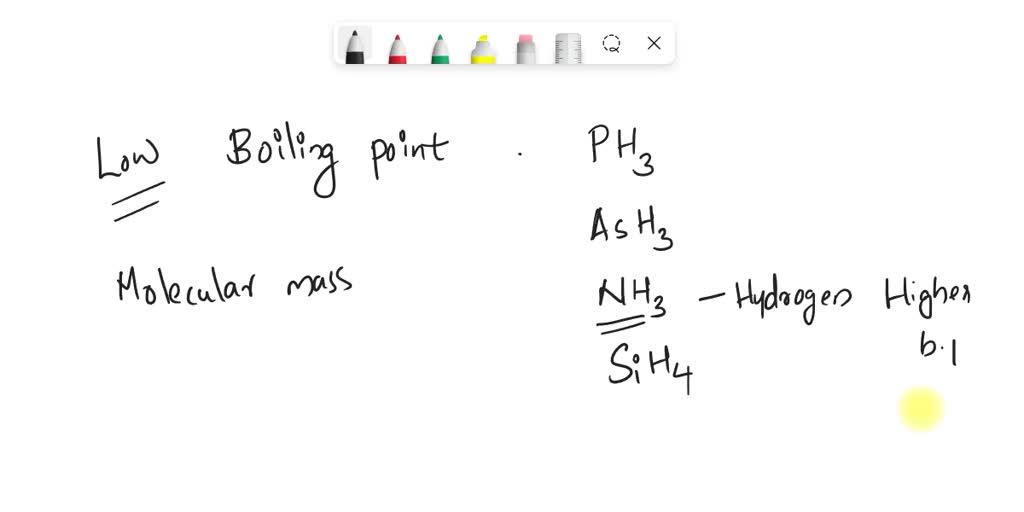
SOLVED: Question 6 1 pts Arrange NH3, CF4, and PH3 in order from lowest to highest boiling points CF4 PH3 NH3 CF4 NH3 PH3 PH3 NH3 CF4 NH3 CF4 PH3 PH3 CF4
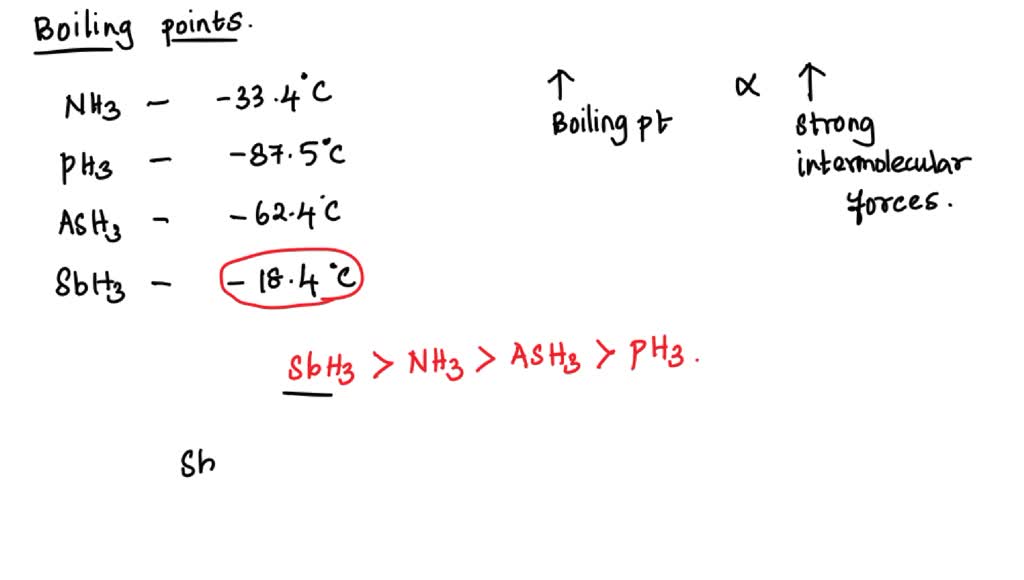
SOLVED: The boiling point of NH3, PH3,AsH3 and SbH3 are respectively -33.4 oC,-87.5 oC, -62.4 oC, -18.4oC. Explain the variation of their boiling points in terms of the types of intermolecular forces.

The hydrides of group 5A are NH3, PH3, AsH3, and SbH3. Arrange them from highest to lowest boiling point. - Brainly.com

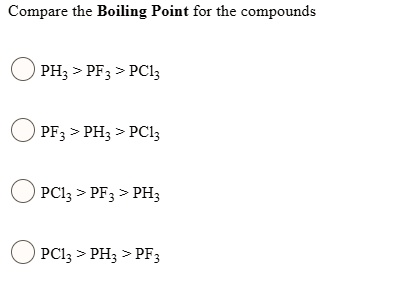
Among the following, which has the highest boiling point? (A) NH3 (B) PH3 (C) AsH3 (D) CH - Brainly.in

The boiling point of phosphine, PH3 (-88 degrees C) is lower than that of ammonia, NH3 (-33 degrees C) even though phosphine has twice the molar mass of NH3. Why? | Homework.Study.com

No links please Q NH3 has higher boiling point than PH3, explain - Chemistry - Solutions - 12503991 | Meritnation.com

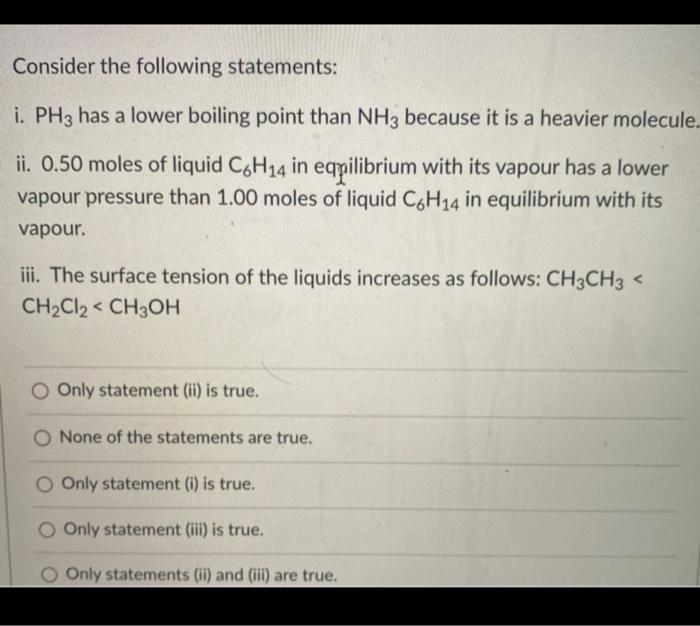


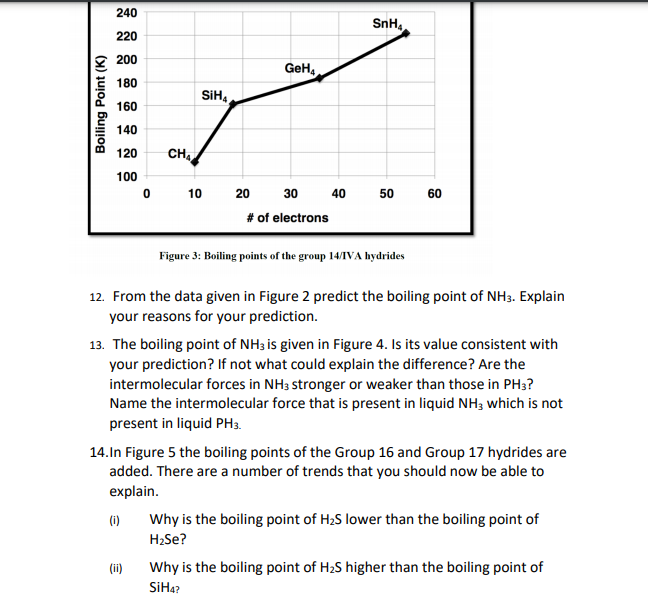
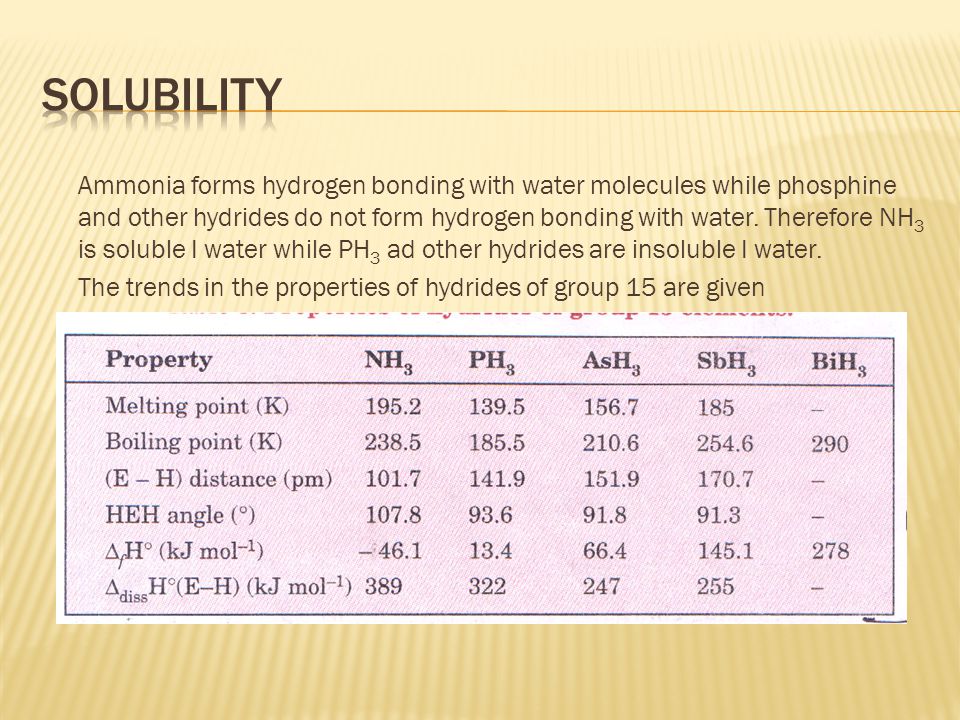
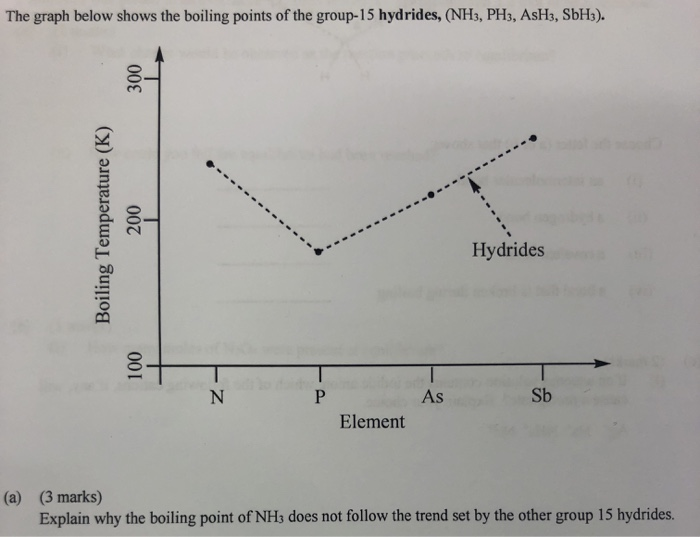



![Solved] NH3 has a much higher boiling point than PH3 because Solved] NH3 has a much higher boiling point than PH3 because](https://storage.googleapis.com/tb-img/production/21/01/F1_Utkarsha.S_30-01-21_Savita_D13.png)