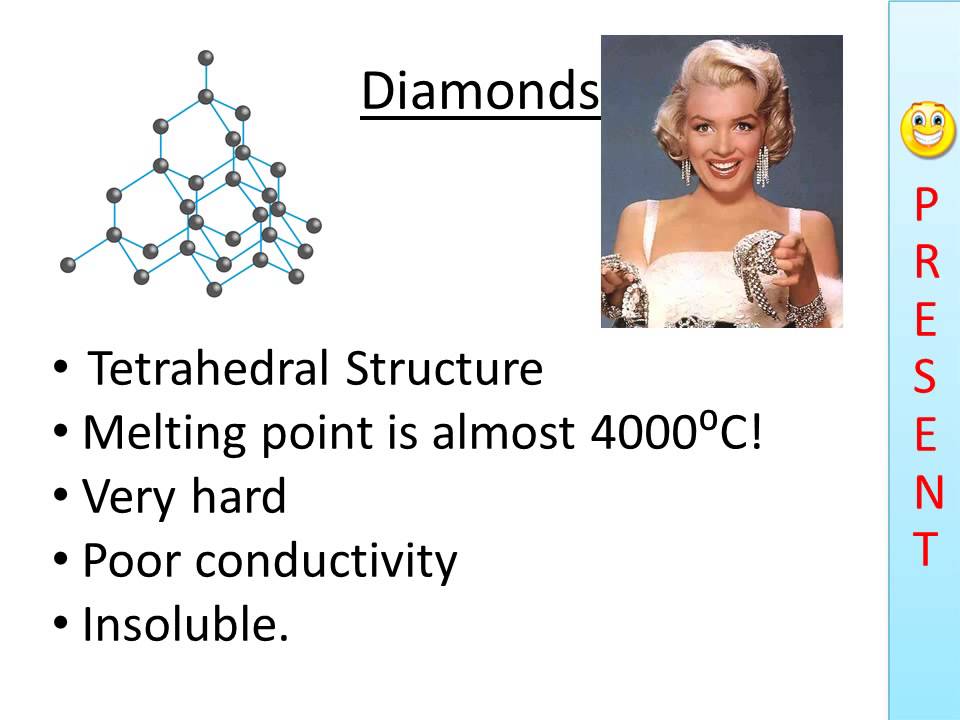
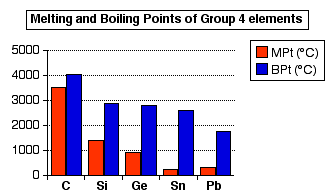
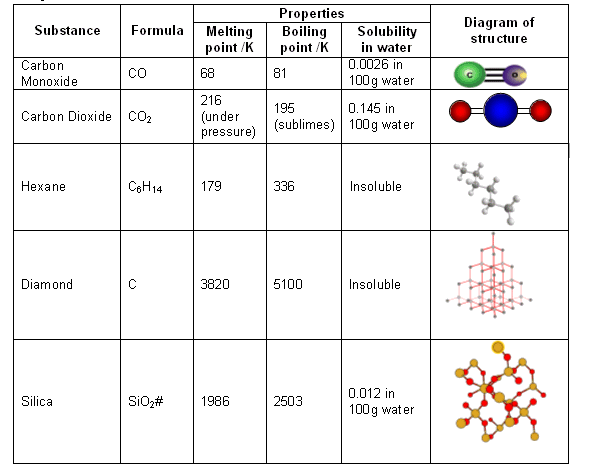
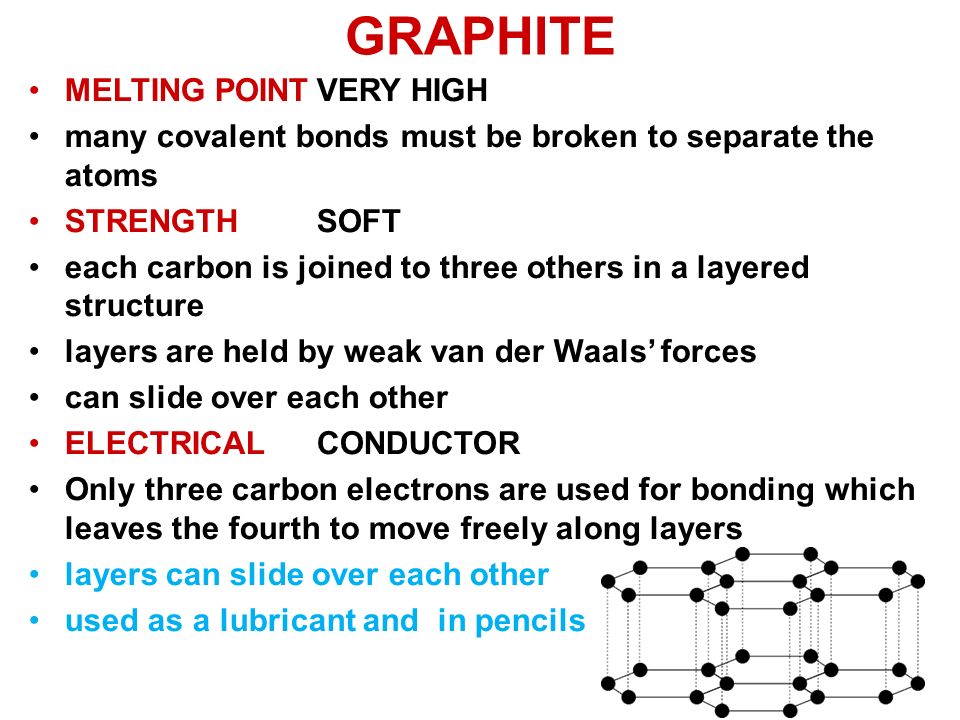
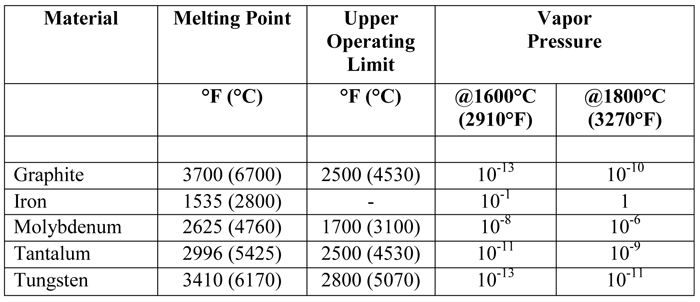
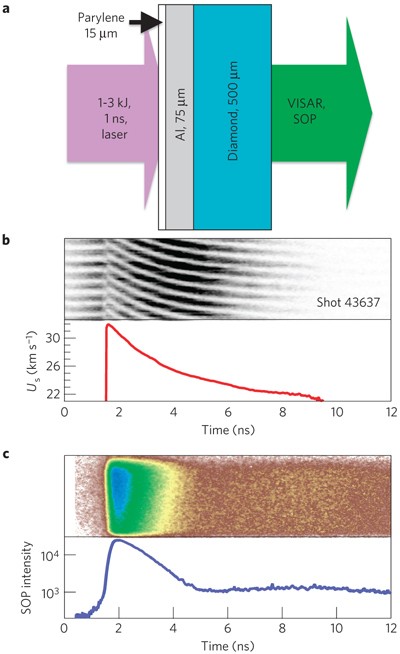
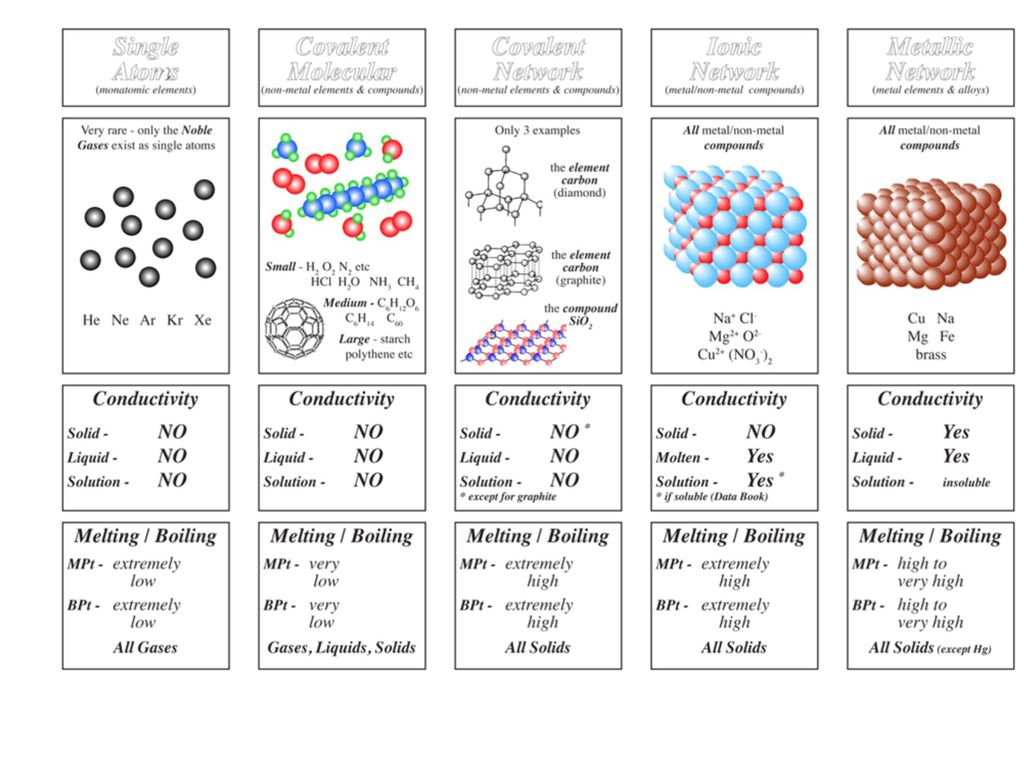
Melting point (mp) - solid to liquid Boiling point (bp) - liquid to gas Volatility - how easily it is converted to gas Conductivity (conducts. - ppt download

THE BEST SCIENTIST IS OPEN TO EXPERIENCE AND BEGINS WITH ROMANCE - THE IDEA THAT ANYTHING IS POSSIBLE. - Ray Bradbury – DON'T SAY YOU DON'T HAVE ENOUGH. - ppt download

Formula: Ir Melting Point: 2410ºC Boiling Point: 4130ºC State: solid Electrical conductivity: conductor Magnetism: non magnetic. - ppt download
How hard is it to melt diamond? Can I make profit melting many little diamonds into one big diamond? I've heard scientists have melted diamond at high temperature and pressures with strong

Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003) - ScienceDirect

Amazon.co.jp: 5 pcs, carbon graphite sheet, high temperature resistance high purity graphite plate, electrode electrolytic plate, 8mm thick, 47.5mm wide, 101mm long : DIY, Tools & Garden

Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003) - ScienceDirect
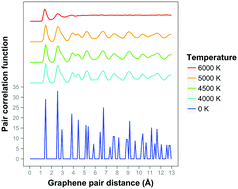
The initial stages of melting of graphene between 4000 K and 6000 K - Physical Chemistry Chemical Physics (RSC Publishing)
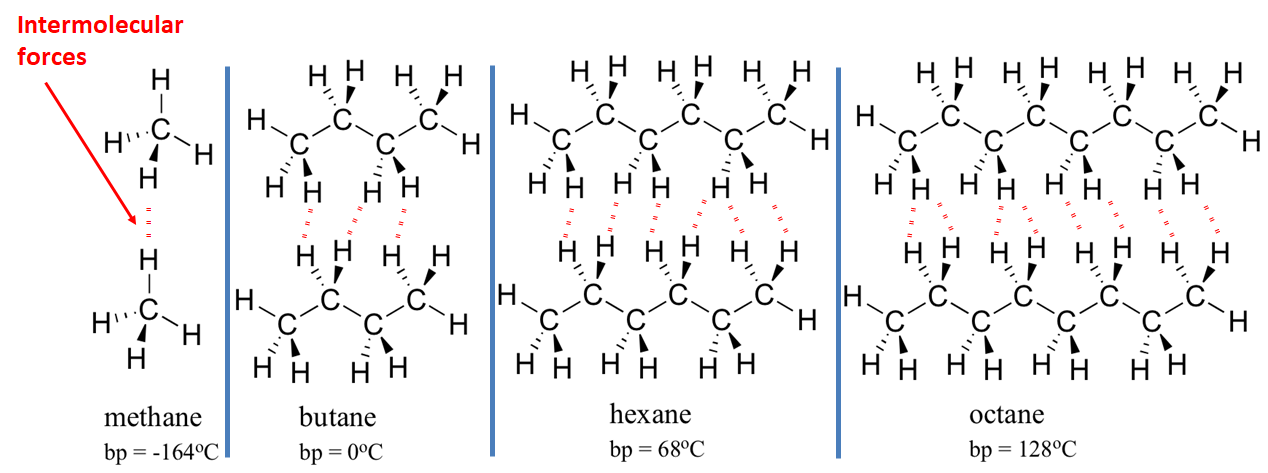
1:48 explain why the melting and boiling points of substances with simple molecular structures increase, in general, with increasing relative molecular mass - TutorMyself Chemistry