Sciencemadness Discussion Board - Hydrogen Peroxide - Illustrated Practical Guide - Powered by XMB 1.9.11

Which of the following is not a proper match(A) D-D is equal to H-H........ Bond length(B) H20 Ꭰ20 , H2O2 - Brainly.in

Molecular solids generally have low melting points and boiling points due to relatively weak intermolecular attractions that hold the molecules in their solid form.Molecular SolidBoiling Point (^∘C) H2Te 0 H2Se - 50
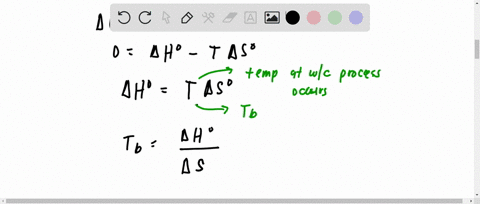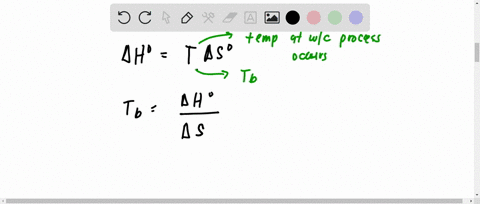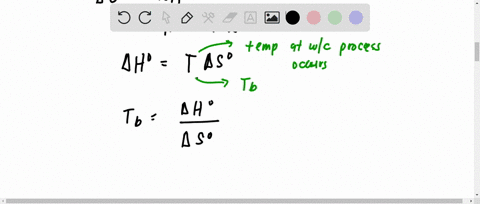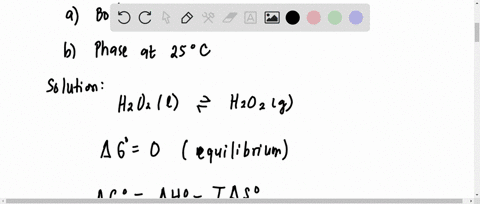
SOLVED:Hydrogen peroxide, H2 O2, has a normal boiling point of 150^∘ C. Based on the data given in Figure 11.25 , would you expect hydrogen peroxide to have a higher or lower

a) Mention conjugate base of each of the following: HS^-,H3O^+,H2PO4^-,HSO4^-,HF,CH3COOH,C6H5OH,HClO4,NH4^+ (b) Mention the conjugate acid of each of the following: OH^-,CH3COO^-,Cl^-,CO3^2 - ,H2PO4^-,CH3NH2,CH3COOH,NH2^- (c) Which of the following ...

SOLVED:Direct measurement of the normal boiling point of hydrogen peroxide is not possible because pure H2 O2 explodes on heating. The boiling point can be estimated, however, from vapor-pressure data. Use the
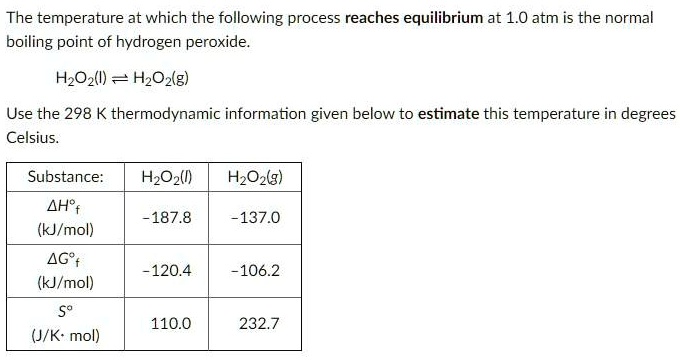










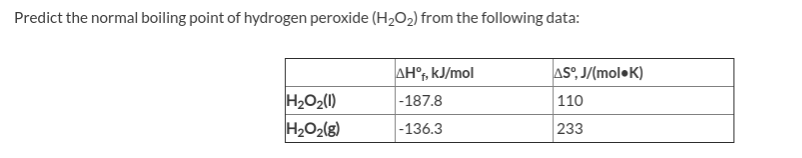

.gif)


