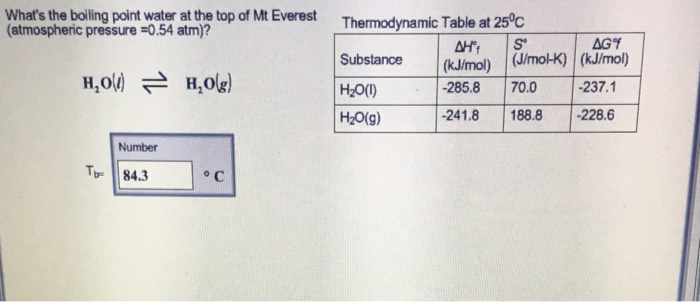
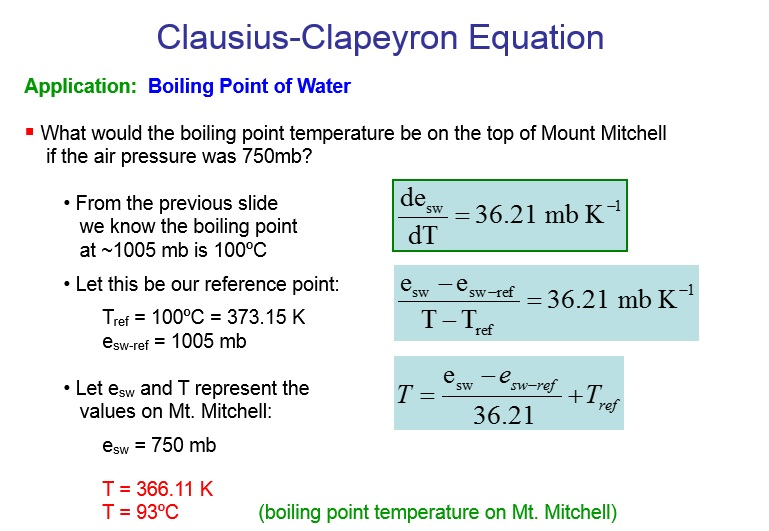
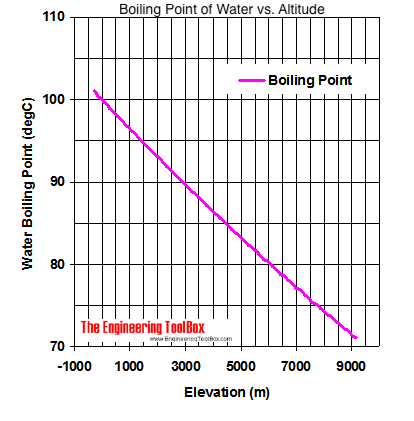
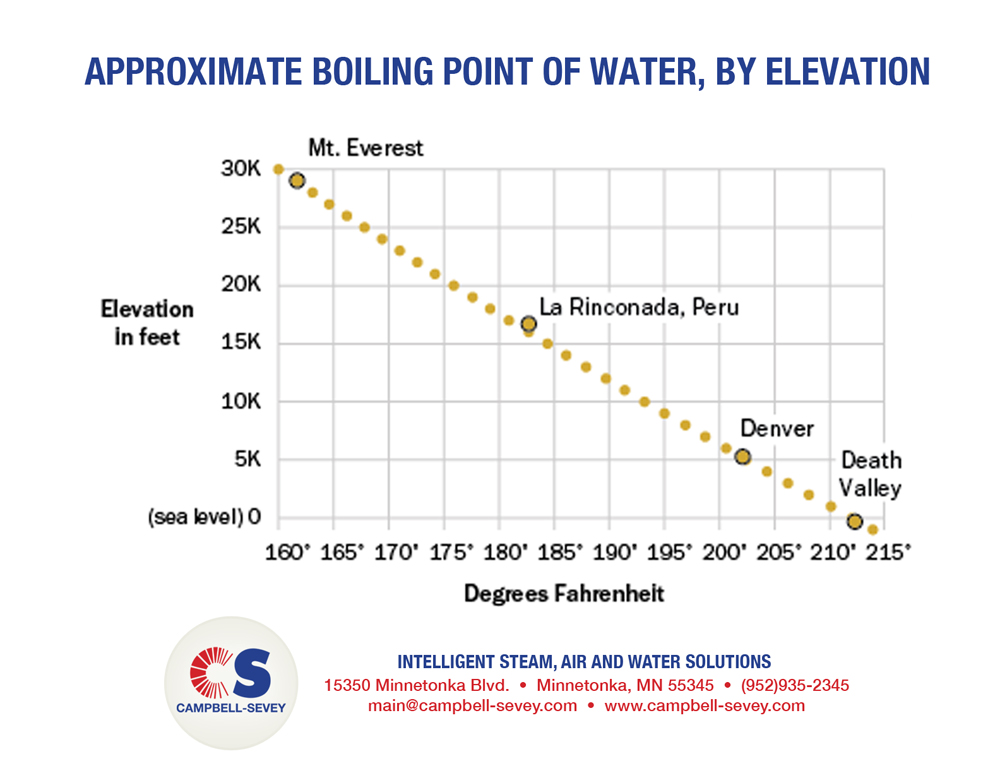
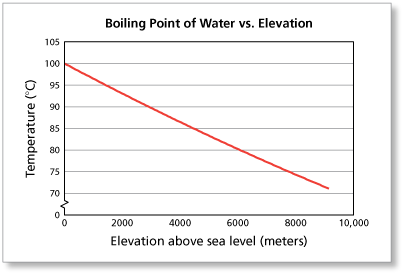
SOLVED: The summit of mount Everest is at 29,029 feet At this elevation the atmosphere pressure is 30 kPa or about 0.30 atm. What is the boiling point of' mount Evcrest? water

Facts | News | Amazing Stuff on Instagram: “What is the boiling temperature of water on Mount Everest?” | Everest, Mount everest, Water

SOLVED:a. Atmospheric pressure on Mount Everest is 224 mm Hg. What is the boiling point of water there? b. What is the freezing point of water on Mount Everest?

Horter Investment Management, LLC - "The boiling point for water at the top of Mt. Everest is 162°F, about 50 degrees below the boiling point at sea level." #FunFactFriday | Facebook

What is the atmospheric pressure on top of Mt. Everest on a day when water boils there at a temperature of 70.0 degree C? | Homework.Study.com

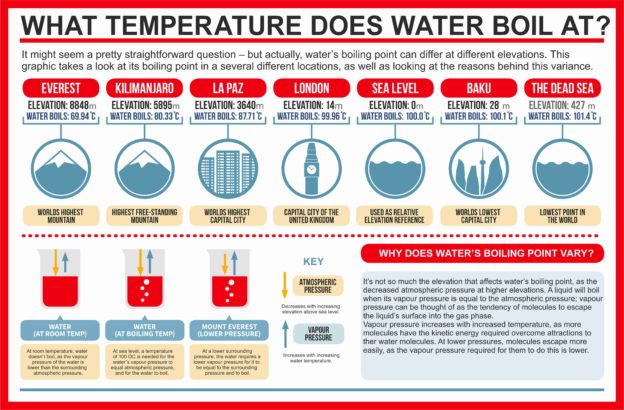
What temperature does water boils at? #boilingpoint #water #bp #mounteverest #kilimanjaro #deadsea #sealevel #t… | Water boiling, Sea level, Science and technology





:max_bytes(150000):strip_icc()/BoilingWater-58dd1c2a5f9b5846837d2a23.jpg)