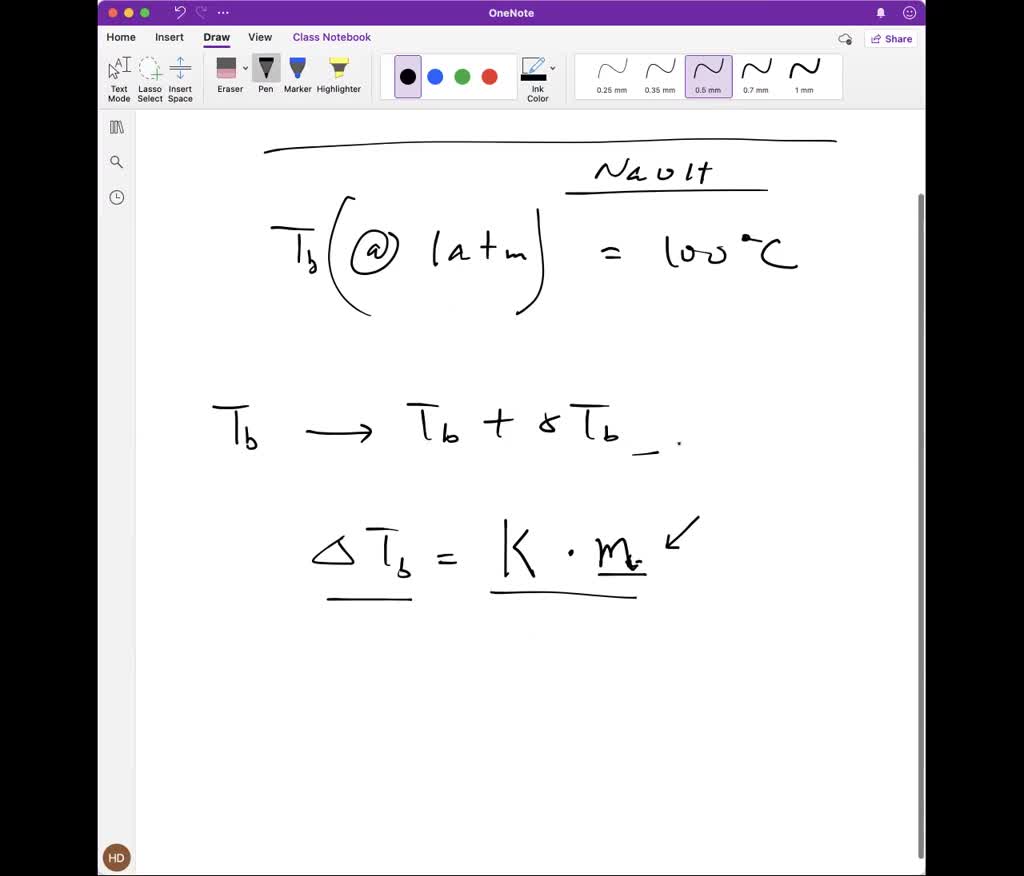
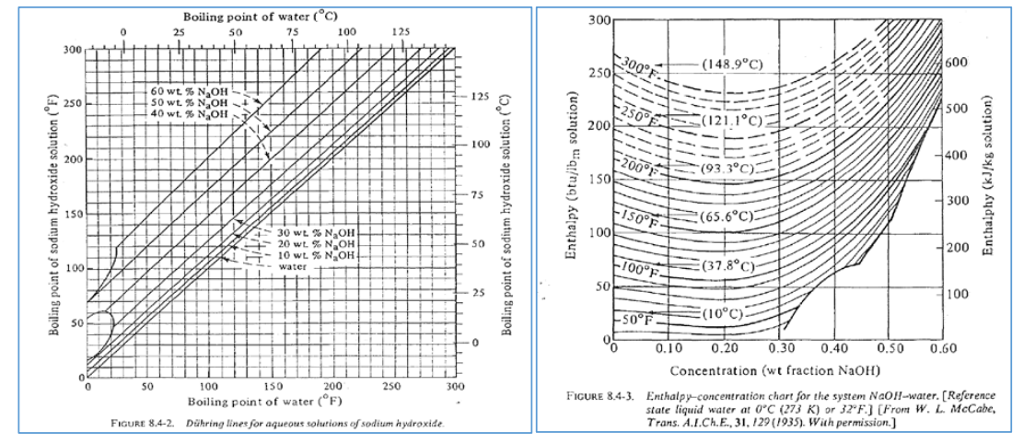
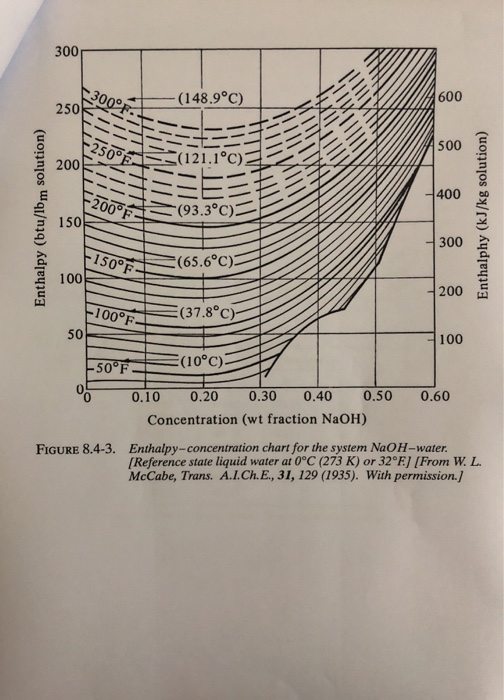
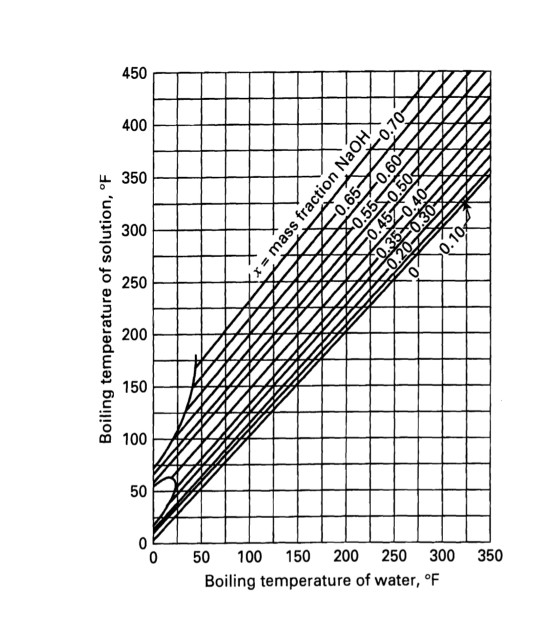
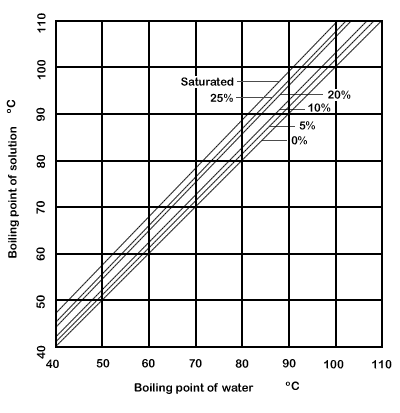
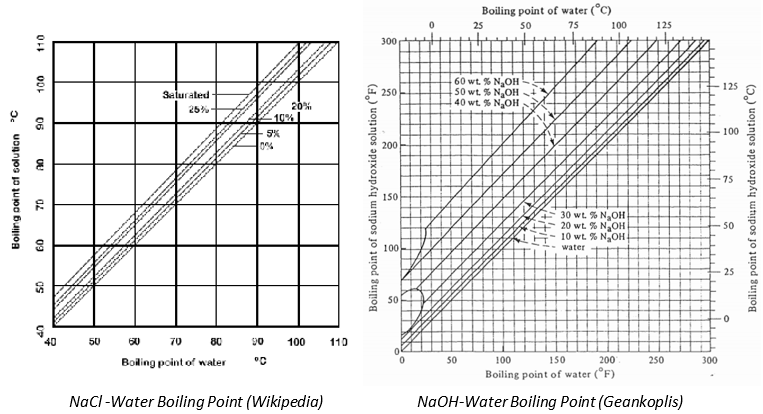
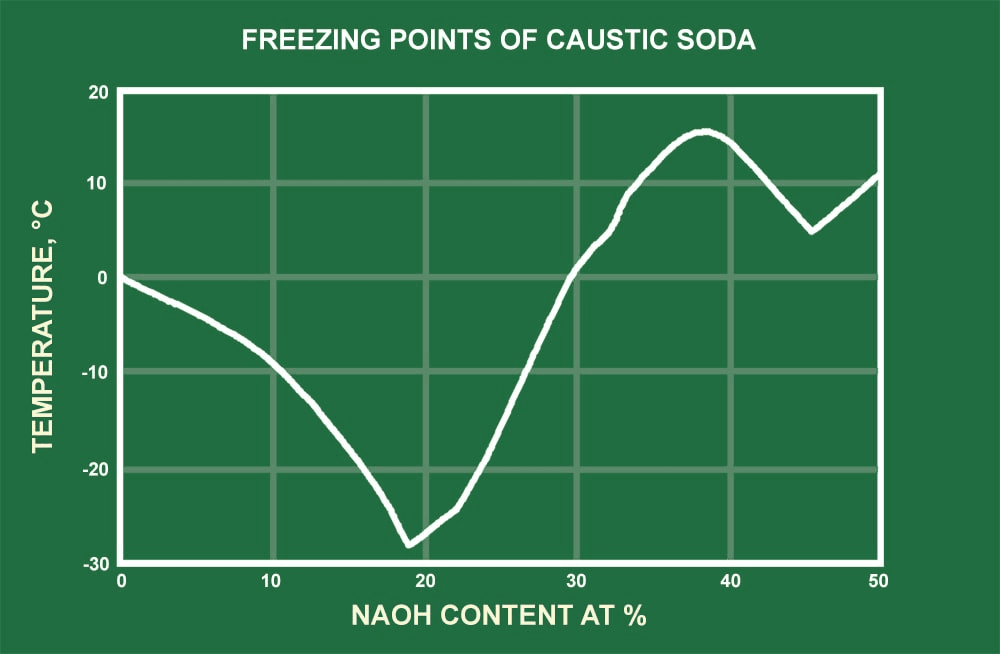
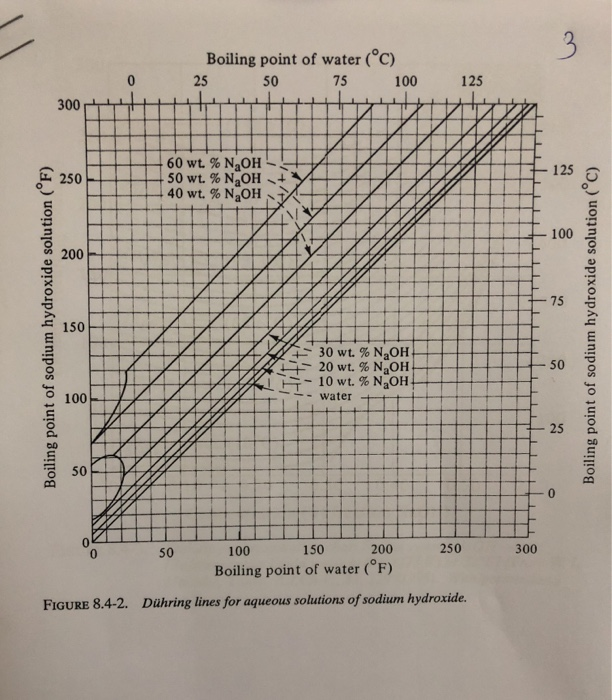
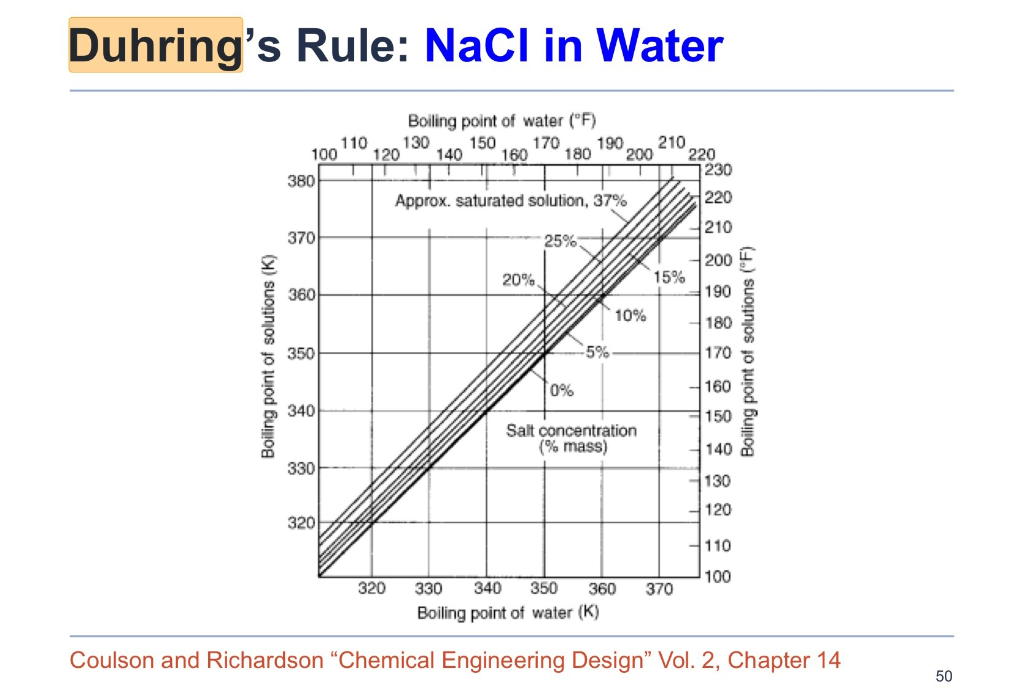
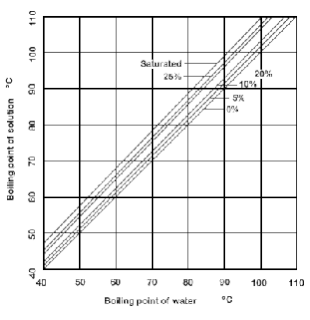
SOLVED: 20.0 g of NaOH was dissolved in 200.0 g of water, calculate the boiling point of the solution. Kb for water is 0.51 C/m

Boiling temperature at atmospheric pressure of aqueous solutions of... | Download Scientific Diagram

Selection of stainless steels for handling sodium hydroxide (NaOH) – British Stainless Steel Association

1: Saturation temperatures for sodium hydroxide solutions for different... | Download Scientific Diagram

Figure 3 from Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method | Semantic Scholar

Boiling temperature at atmospheric pressure of aqueous solutions of... | Download Scientific Diagram

Boiling temperature at atmospheric pressure of aqueous solutions of... | Download Scientific Diagram