Pentane has a boiling point of 36.1 degrees Celsius while 1-butanol, which has a similar mass, has a boiling point of 117.7 degrees Celsius. Explain this difference, including line-angle structures of each
Why Isomers of a compound have different Boiling point (like Isomers of pentane) why force of attraction is not involve in it? - Quora

SOLVED: 1)The following information is given for n-pentane, C5H12, at 1atm: boiling point = 36.2 °C Hvap(36.2 °C) = 25.8 kJ/mol specific heat liquid = 2.28 J/g°C At a pressure of 1

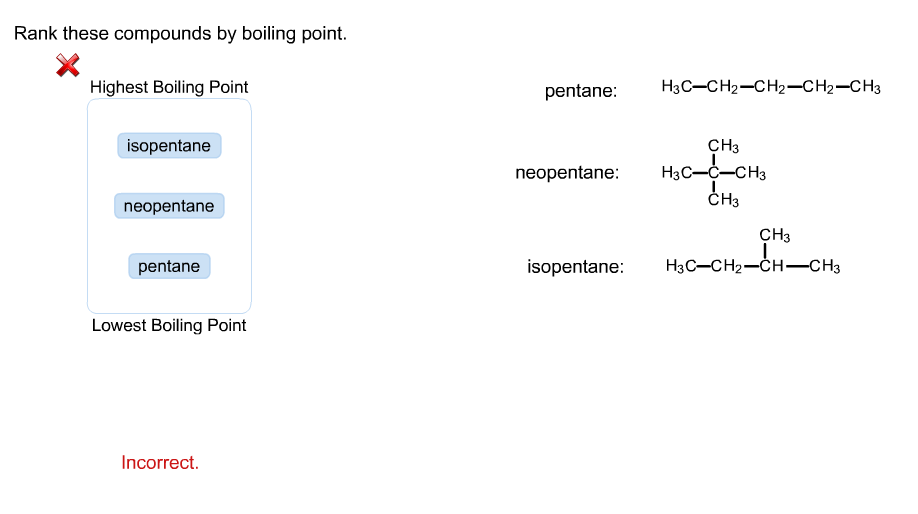
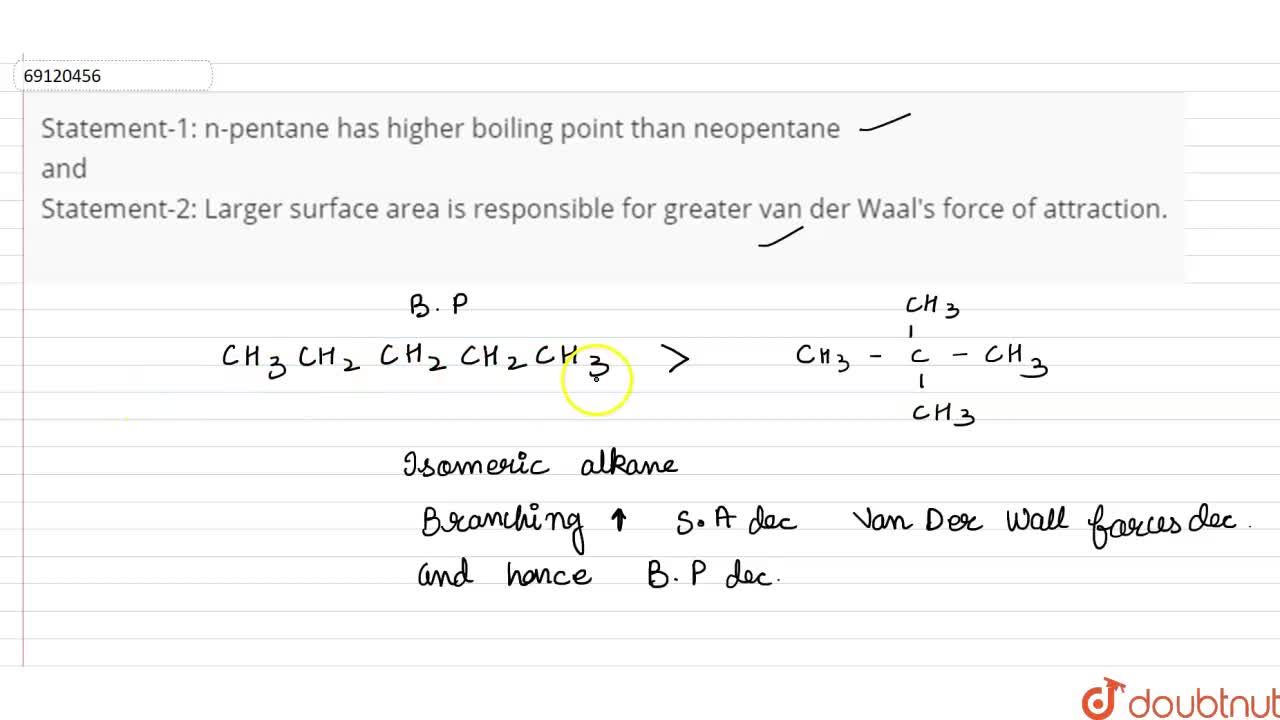
Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane
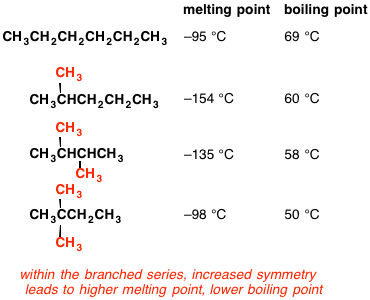
Why is the melting point of neopentane higher than n-pentane but the melting point of isopentane lower than that of n-pentane? Why is the order different than that of their boiling points? -

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange
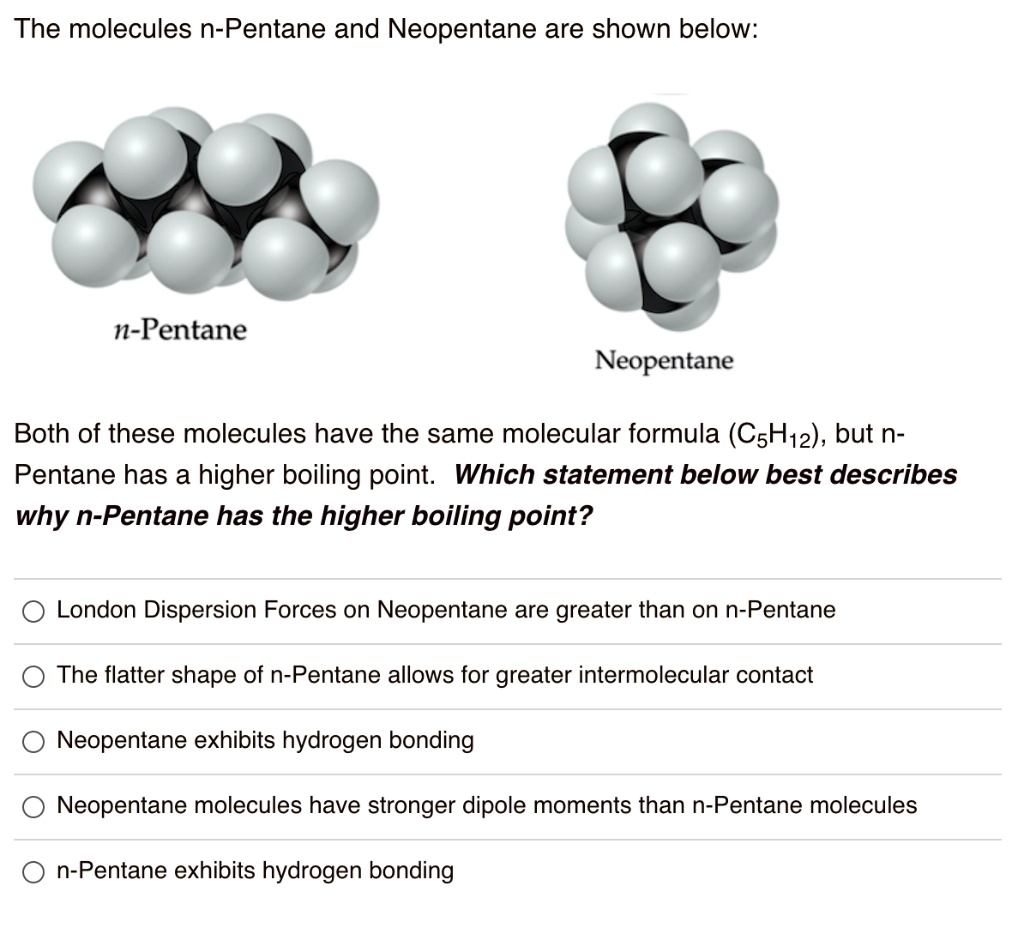
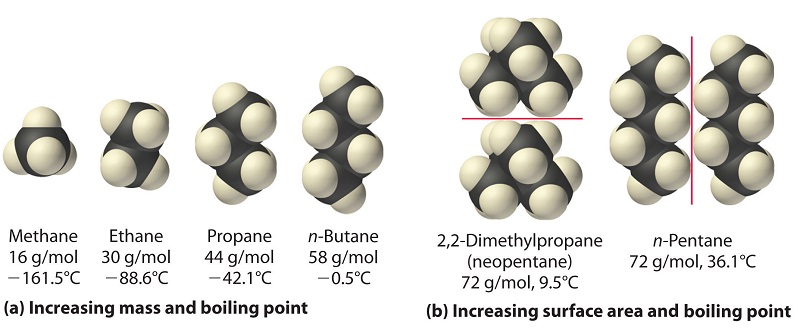
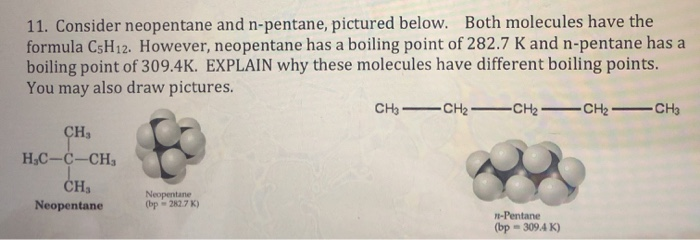
SOLVED: The molecules n-Pentane and Neopentane are shown below: n-Pentane Neopentane Both of these molecules have the same molecular formula (C5H12), but n- Pentane has a higher boiling point: Which statement below

Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com