1:49 explain why substances with giant covalent structures are solids with high melting and boiling points - TutorMyself Chemistry
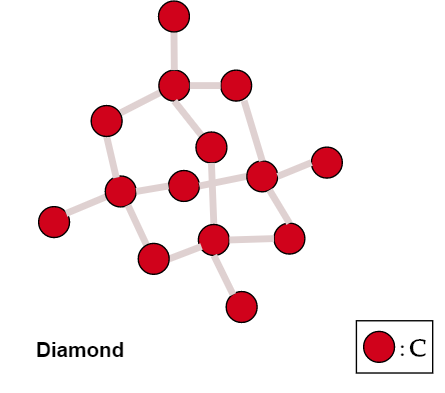
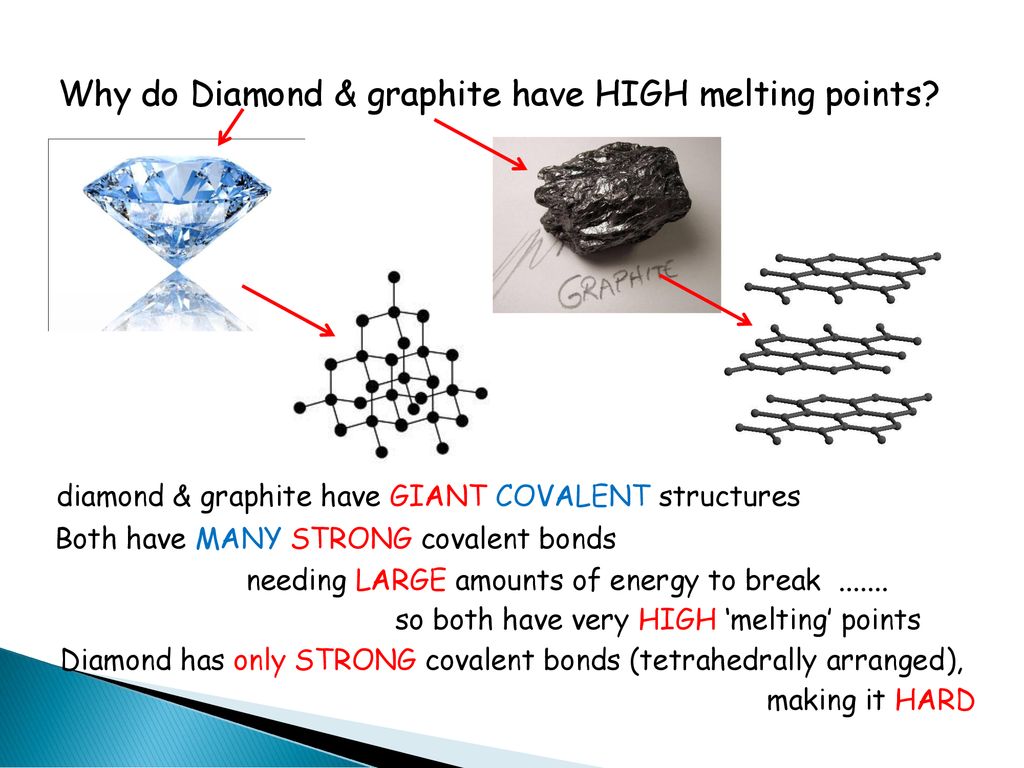
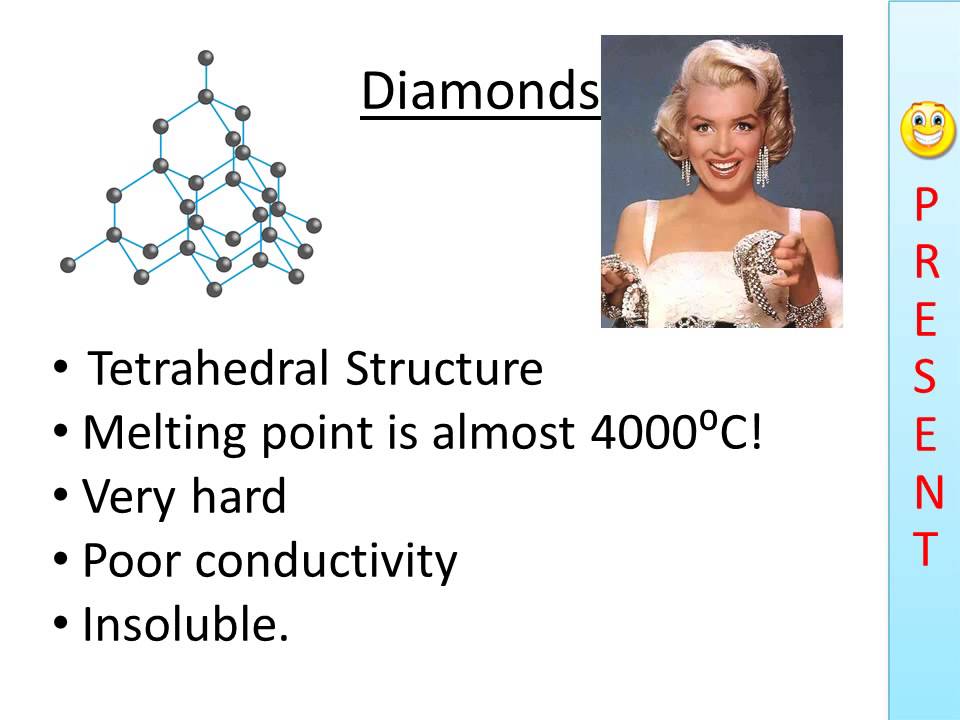
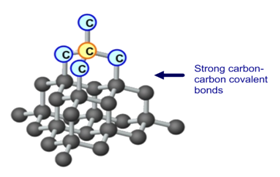
The high melting and boiling points of diamond are due to:A. Its high refractive indexB. Its high IP valueC. Giant polymer structure with strong covalent bondsD. Its high electronegativity
How hard is it to melt diamond? Can I make profit melting many little diamonds into one big diamond? I've heard scientists have melted diamond at high temperature and pressures with strong

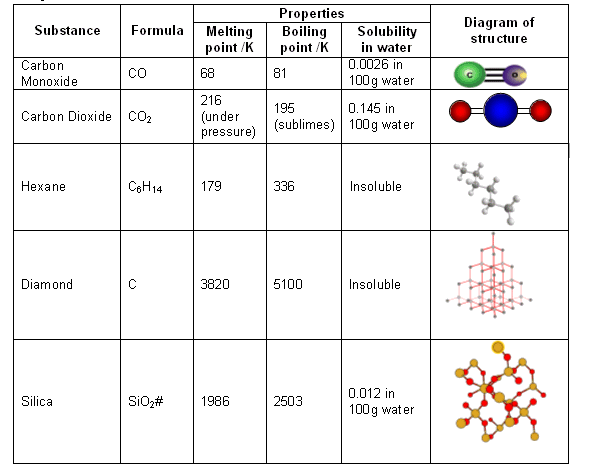
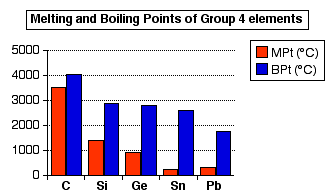
DIAMOND FACT 21 Diamonds have very large melting point of 3820K (3547°C / 6420F) and a boiling point of 5100K (4827°C / 8720F) | Diamond facts, Informative, Facts