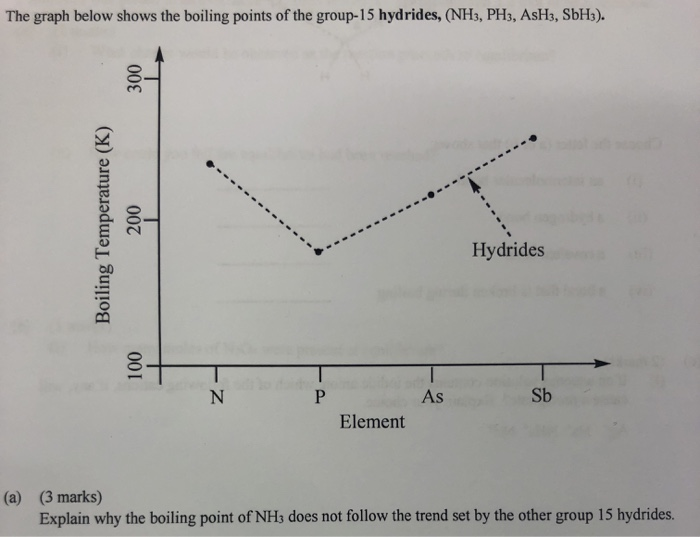
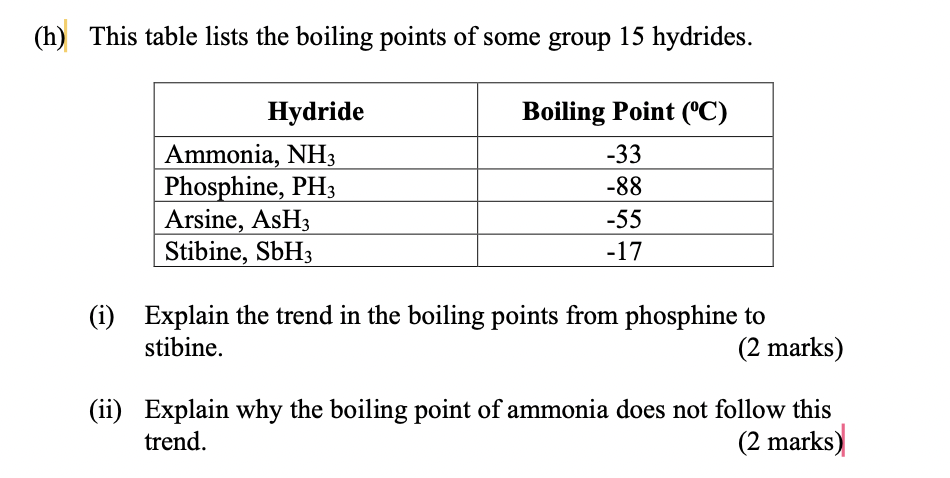
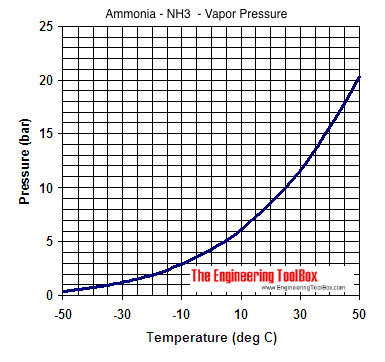
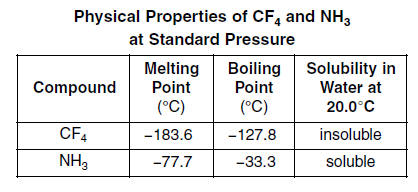
SOLVED: The normal boiling points of ammonia, water, and hydrogen fluoride increase in the order NHs (bp = -33 C) < HF (bp 19 C) HzO (bp = 100 oC): What is

home experiment - Does the boiling point of ammonia hydroxide change with the ratio of water to ammonia? - Chemistry Stack Exchange





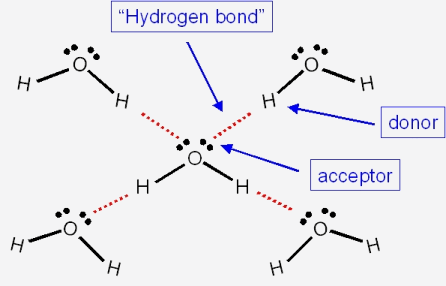


![Solved] NH3 has a much higher boiling point than PH3 because Solved] NH3 has a much higher boiling point than PH3 because](https://storage.googleapis.com/tb-img/production/21/01/F1_Utkarsha.S_30-01-21_Savita_D13.png)