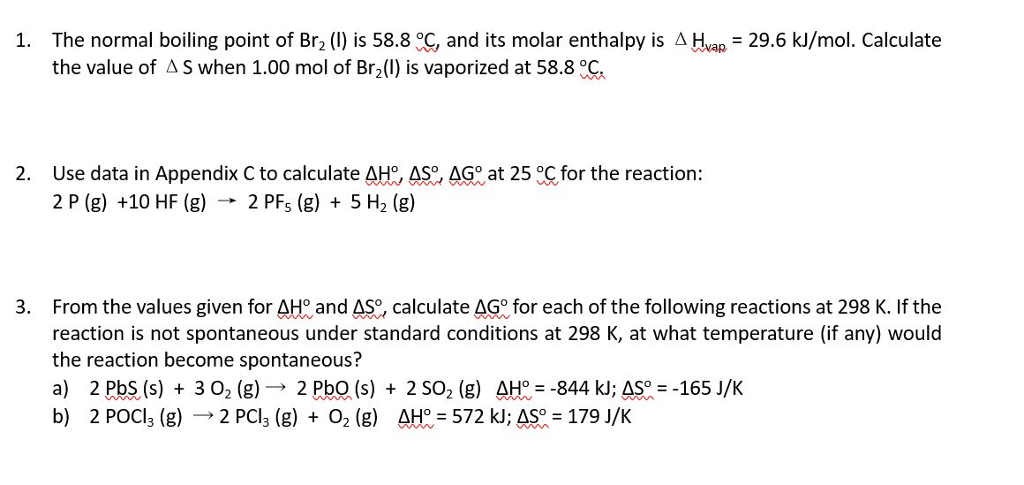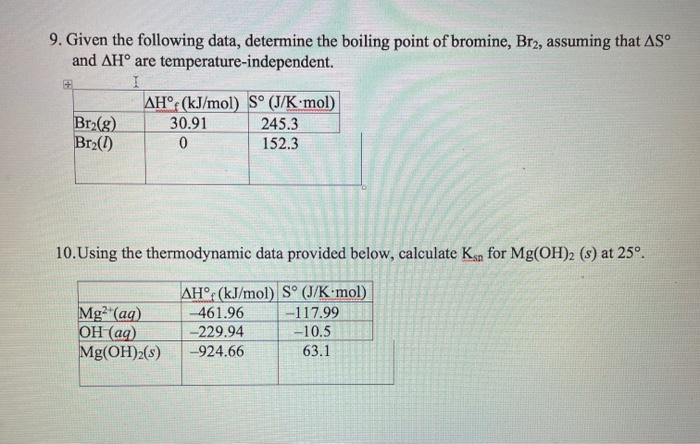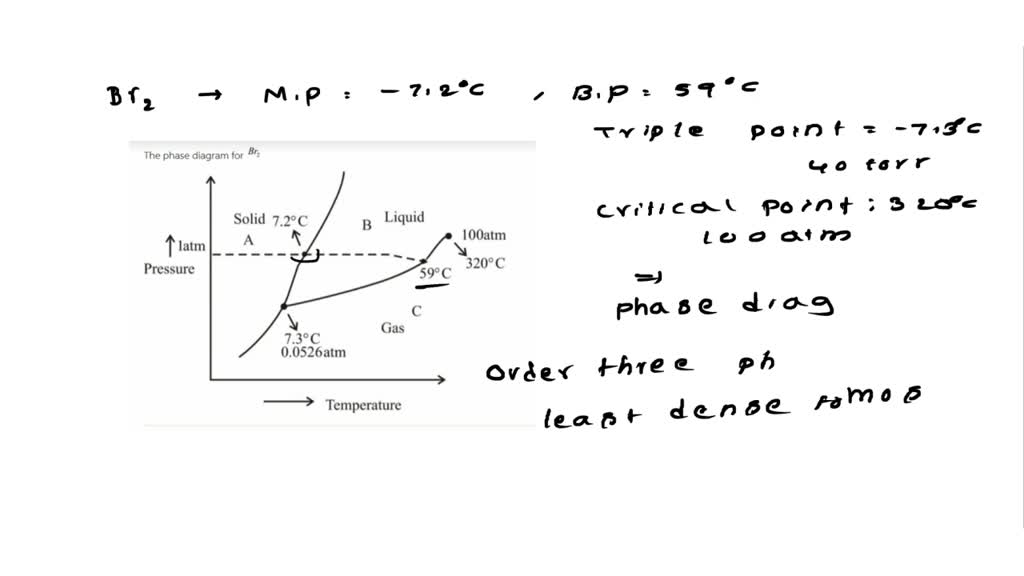
SOLVED: Bromine (Br2) has a normal melting point of – 7.2°C and a normal boiling point of 59°C. The triple point of Br2 is – 7.3°C and 40 mm Hg, and the

SOLVED: 1- If ΔHvap of Bromine (Br2) is 31 kJ/mol, and the ΔSvap of Br2 is 93 J/(mol·K), what is the boiling point of Br2 in oC? Hint 1: What is the

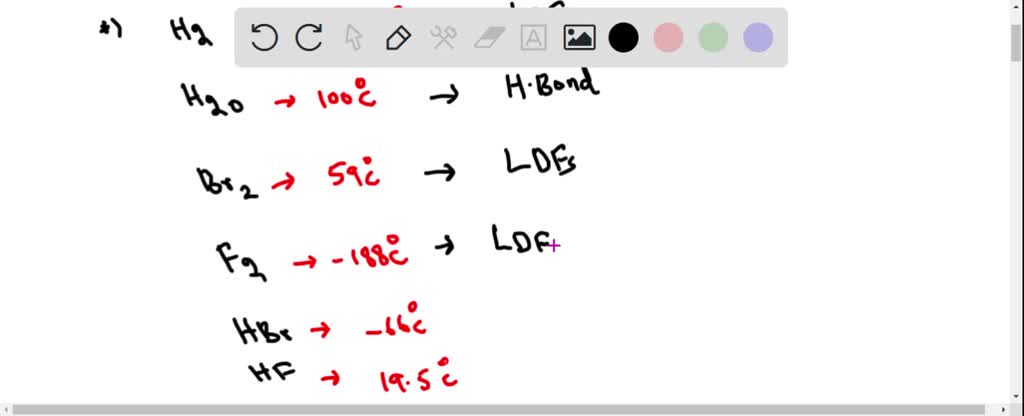
SOLVED: Identifythe moelcule you expect to have the highest boiling point? OA F2| 0 B Cl2 C Br2 0D. I2 OE All of the above have the same boiling point

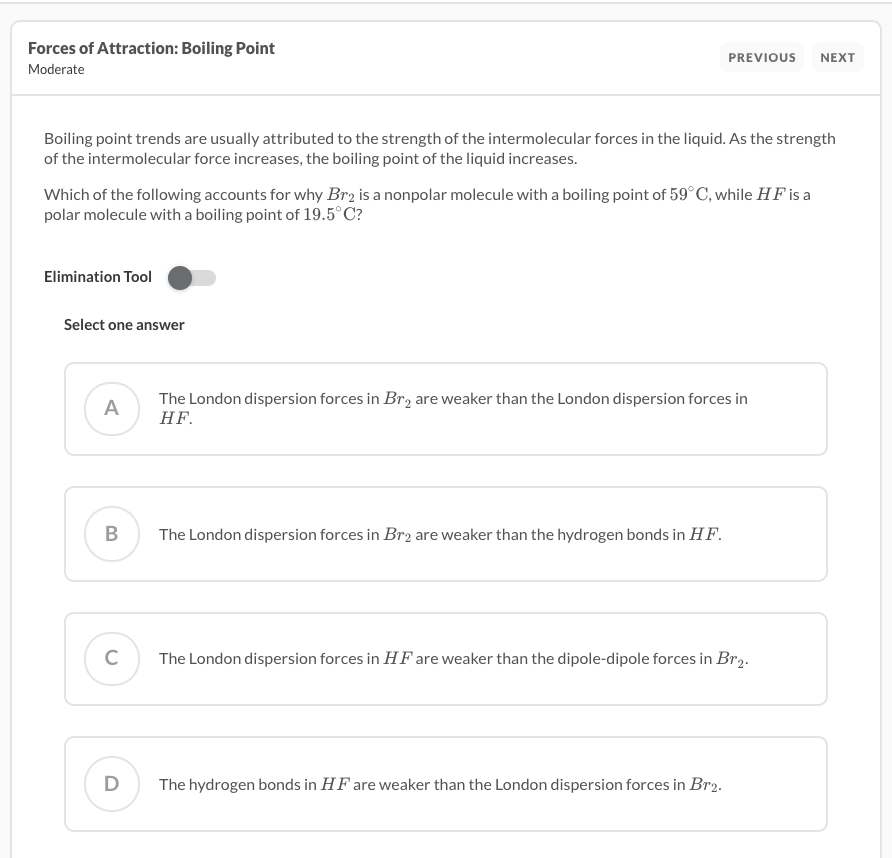
SOLVED: Explain why the boiling point of Br2 (59°C) is lower than that of iodine monochloride, ICl (97°C), even though they have nearly the same molar mass.Choose one: A. Br2 is larger
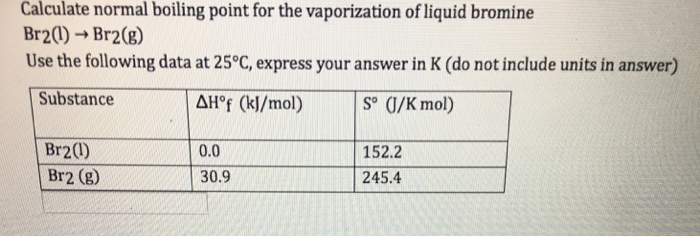
![SOLVED:The normal boiling point of bromine is 58.8^∘ C, and the standard entropies of the liquid and vapor are S^∘[Br2(l)]=152.2 J /(K ·mol) ; S^∘[ Br2(g)]=245.4 J /(K ·mol) . At what temperature SOLVED:The normal boiling point of bromine is 58.8^∘ C, and the standard entropies of the liquid and vapor are S^∘[Br2(l)]=152.2 J /(K ·mol) ; S^∘[ Br2(g)]=245.4 J /(K ·mol) . At what temperature](https://cdn.numerade.com/previews/d652b980-3ccd-43e0-bc66-803c30367837_large.jpg)
SOLVED:The normal boiling point of bromine is 58.8^∘ C, and the standard entropies of the liquid and vapor are S^∘[Br2(l)]=152.2 J /(K ·mol) ; S^∘[ Br2(g)]=245.4 J /(K ·mol) . At what temperature
![Q102E Like most substances, bromine ex... [FREE SOLUTION] | StudySmarter Q102E Like most substances, bromine ex... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/image_ntfOxYQ.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230602%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230602T221832Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=12fb6b94416bdb3ae58c270dec64d1fafffdc37f343b49e1cdc623eed47f7f46)